The import and distribution of pharmaceuticals within the European Union (EU) are governed by a stringent regulatory system designed to ensure that patients across the EU have access to high-quality, effective, and safe medicines. Within this framework, before any medicinal product can be placed on the EU market, it must undergo a rigorous authorisation procedure. A critical yet often overlooked component of this procedure is the Linguistic Review of Product Information translations. This article explores the key steps involved in this process for pharmaceuticals for human use, authorised for the European Single Market via the Centralised Procedure.
What Is the Marketing Authorisation Process for Medicinal Products in the EU?
The marketing authorisation process for medicinal products in the EU is governed by a uniform regulatory framework designed to ensure that only safe and effective medicines reach the market. This framework operates under Regulation (EC) No 726/2004, which establishes the legal basis for the marketing authorisation of medicinal products for human use across the European Economic Area (EEA). The EEA, also known as the European Single Market, consists of the 27 EU Member States as well as non-EU countries of Iceland, Liechtenstein, and Norway, which are required to incorporate relevant EU legislation into their national laws to have access to the European Single Market.
The system relies on a network of approximately 50 regulatory authorities, including the European Medicines Agency (EMA), the European Commission, and the national regulatory bodies of the 30 EEA countries. This collaborative approach facilitates the homogeneous application of a single set of common rules across the entire EEA.
The marketing authorisation process is structured around four primary routes, each catering to different types of products and their specific needs:
- Centralised Procedure (CP)
- Decentralised Procedure (DCP)
- Mutual Recognition Procedure (MRP)
- National Procedure
What Is the Centralised Procedure?
The Centralised Procedure (CP) allows for the marketing of a medicinal product based on a single assessment and authorisation that is valid throughout the EEA.
Pharmaceutical companies initiate the CP by submitting a single marketing authorisation application (MAA) to EMA. This MAA undergoes a rigorous scientific assessment by EMA’s Committee for Medicinal Products for Human Use (CHMP). Following this assessment, the CHMP issues a recommendation to the European Commission, which makes the final, legally binding decision on whether to grant or refuse the marketing authorisation. Once granted, the centralised marketing authorisation automatically becomes valid across the EEA, eliminating the need for any additional national authorisations. The MAA process is conducted in English, which is EMA’s official working language.
The CP is mandatory for certain categories of medicines, particularly innovative therapies for unmet medical needs, including rare diseases, and advanced-therapy products that require expedited reviews due to their significant impact on patient care. By streamlining the authorisation mechanism, the CP not only enhances market access for new medicines but also ensures comprehensive evaluations that uphold high public health and safety standards.
What is the Product Information?
For centrally authorised products (CAPs), the Product Information (PI) is a crucial document that provides officially approved information for healthcare professionals (HCPs) and patients regarding a medicinal product.
The PI comprises the following key components:
- Summary of Product Characteristics (SmPC or SPC)
This serves as the foundational document for HCPs, detailing the product’s properties and how to use it safely and effectively within the scope of the marketing authorisation. - Annex II
This section reflects the CHMP opinion, detailing specific conditions and obligations imposed on the marketing authorisation. - Labelling
This includes text used for the immediate or outer packaging of the product, such as blister foil, bottle labels, or cartons. - Package Leaflet (PIL)
This presents information aligned with the SmPC but written in lay terms for end-users, such as patients, nurses, or caregivers. A printed copy is enclosed with the product supplied for sale.
Final version of the PI must be submitted to EMA as a single document.
During an initial MAA, the English Product Information (EN PI) is prepared by the applicant in accordance with Quality Review of Documents (QRD) templates and stylistic requirements, as well as regulated terminology from EDQM Standard Terms and the Medical Dictionary for Regulatory Activities (MedDRA). The EN PI then undergoes multiple rounds of revision by EMA. All comments must be implemented when the revised EN PI is submitted for a final check before the CHMP Opinion, at which point the final version of the EN PI is approved.
What is EMA’s Linguistic Review of Product Information?
The Linguistic Review of PI involves the review and approval of 24 national language versions of the PI by the corresponding EU-EEA Member States. This standardised process is conducted within a set timeline of 14 calendar days and applies to all marketing authorisations, extensions, and other post-authorisation procedures affecting the PI.
Within 5 calendar days of a positive CHMP Opinion, the Marketing Authorisation Holder (MAH) submits to EMA the final EN PI, along with its translations (also referred to as national texts) into the 24 other EU-EEA languages. These include the 22 official EU languages plus Icelandic and Norwegian. Although Irish was reinstated as an official working language of the EU in January 2022, it is typically not included in the PI language package for products authorised via the Centralised Procedure.
EMA then initiates the Linguistic Review period and distributes the national texts to the individual EU-EEA Member States for review. The Member States have 14 calendar days to review the national texts and return the tracked changed documents with their comments directly to the MAH.
For New Marketing Authorisation Applications and Extensions
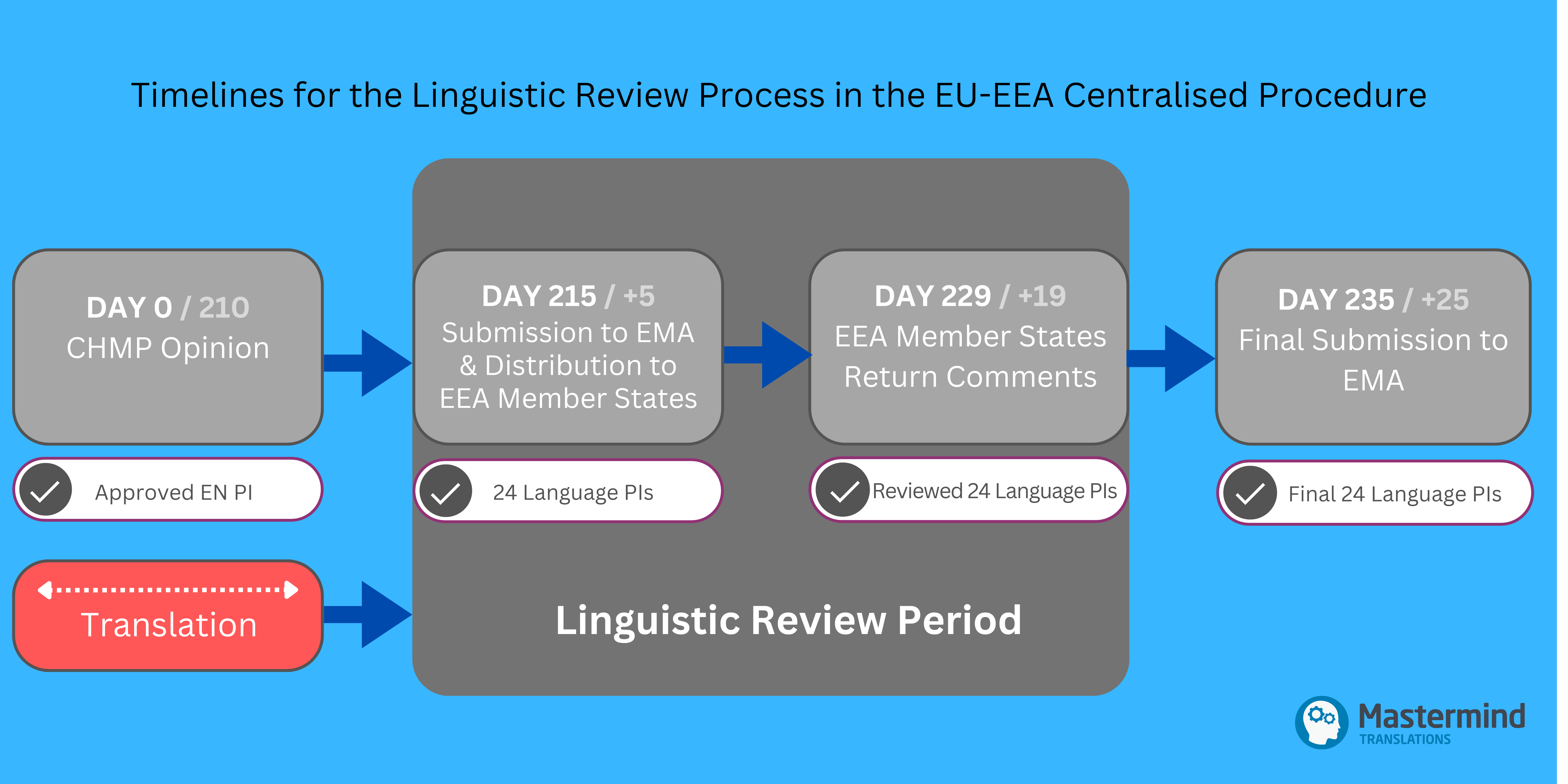
The submission of the initial MAA to EMA, which includes the first draft of the EN PI, marks Day 1, when the clock starts. During this assessment stage, the EN PI undergoes several rounds of revision. The key time point is Day 210, when the CHMP issues its Opinion and approves the final EN PI.
The Linguistic Review process starts at Day 215 when the MAH submits to EMA the 24 language versions of the adopted EN PI, and of Annex A, along with QRD Form 1, and a Checklist. EMA then distributes the translations to the individual EU-EEA Member States for detailed review. The Member States are expected to return their comments directly to the MAH by Day 229 when the Linguistic Review process ends.
The MAH then finalises the reviewed language PIs and incorporates any comments and edits as tracked changes for visibility. By Day 235, the MAH must submit to EMA via Eudralink:
- 25 annotated Word and clean PDF PIs (24 languages plus English),
- Annotated Word Annex As (if necessary),
- 25 clean PDF Annex As (24 languages plus English),
- Populated international non-proprietary name table,
- QRD form 2, and
- Annex 127a (if required).
EMA subsequently checks if all comments have been implemented before sending the final translations to the European Commission at Day 237 for Final Decision at Day 277.
For Post-Authorisation Procedures
Post-authorisation procedures involve changes to the terms of a marketing authorisation, and the type of variation determines the extent to which the PI is affected. There are three primary types of variations, each with different implications for the Linguistic Review process:
- Type IA Variations
These are considered minor changes that typically do not affect the PI, so the Linguistic Review may not be required. - Type IB Variations
These are also minor changes but may involve adjustments to specific sections of the PI, such as updates to labelling. In these cases, the Linguistic Review may be required, but the impact is generally minimal. - Type II Variations
These involve more substantial changes that can significantly affect the PI, such as updates to therapeutic indications, dosage, or new safety information. The Linguistic Review is mandatory for Type II variations, as updates to the PI must be reviewed and approved in all EU-EEA languages.
Although the steps may vary slightly depending on the type of variation, the Linguistic Review process is essentially the same across all types.
For Type IA/IB Variations, the clock starts on Day 0, when annotated Word and clean PDF PIs in all EU-EEA languages are included in the submission of the variation package to EMA. EMA validates the submission on Day 7, and the MAH submits the annotated Word PI language files directly to Member States via Eudralink on Day +5. The Linguistic Review is performed by Member States over a 14-calendar day period, with the reviewed files returned to the MAH by Day +19. The MAH should then finalise the translations and submit the final versions (annotated Word files and clean PDFs) by Day +25. This submission should also include Annex A (if applicable) and QRD Form 2.
For Type II Variations, the overall process is similar to that of MAAs. The annotated EN PI is submitted for review, and the CHMP Opinion marks Day 0, starting the clock. The annotated national texts are provided by Day +5, which begins the Linguistic Review process by the Member States. Member States return the reviewed files by Day +19, and the final annotated Word files and clean PDFs, along with QRD Form 2, must be submitted to EMA by Day +25. The final translations are transmitted to the European Commission on Day +27, with the Final Decision made on Day +75. Since Day 0 for Type II variations corresponds to the CHMP Opinion and not the submission of the variation package, the MAH has more flexibility to prepare the translations.
What Are the Key Timelines for Translations?
- Day 215 / Day +5 Submission
Once the CHMP Opinion is adopted, the 24 language PIs must be submitted within 5 calendar days. Since the CHMP meetings typically conclude on a Thursday each month, there are usually only 3 working days to finalise the submission. Given the short timeframe and the complexity of the translation work required, MAHs are strongly advised to initiate the translation process well in advance during the Pre-Opinion stage. This is particularly important for initial MAAs, where a full content translation is needed. Ideally, the Day 121/180 version of the EN PI should be shared with the translation provider to allow them sufficient time to prepare the translations, which can then be finalised after the last update at Day 210. - Day 235 / Day +25 Submission
After the deadline for Member States’ comments on Day 229 / Day +19, there are only 6 calendar days to check and finalise the submission. The MAH must ensure that all feedback from Member States has been incorporated into the translations and submit the final versions by Day 235 / Day +25. This includes both the annotated and clean Word versions of the 24 language PIs.
Best Practices for the Translation of Product Information in Preparation for the EMA Linguistic Review Process in the Centralised Marketing Authorisation Procedure
The translation process for the PI in the EU Centralised Procedure is highly time-sensitive, especially after a positive CHMP Opinion. Below are some key best practices to ensure a smooth and efficient translation process while adhering to the strict timelines set by EMA:
- Partner with an Experienced Language Service Provider (LSP)
Choose an LSP with expertise in regulatory affairs for the EU-EEA and a good understanding of the timelines and procedures followed. They are also more likely to have a vetted pool of pharmaceutical translators familiar with QRD projects. - Communicate Your Requirements and Expectations Clearly
Explain the procedures followed for your product to help the LSP understand your specific requirements and key time points. This ensures they can provide a tailored solution that meets your objectives and aligns with your timeline. - Initiate the PI Translation Early
Start the translation process well in advance of the CHMP Opinion. Early engagement with the LSP allows for thorough preparation, provides ample time for revisions, and ensures the tight schedule is met. - Use a Phased Approach for Language PIs
To ensure a streamlined and organised workflow, support your LSP in updating the language PIs in waves rather than all at once. This will provide sufficient time for each time-sensitive step and help maintain smooth progress. - Maintain a Locked Version of Your EN PI
When you release an EN PI version to your LSP, commit to it until the next round of EMA feedback. Even the smallest tweaks can significantly delay the project, as changes must be communicated to all 24 translators, complicating version management and consuming valuable time.